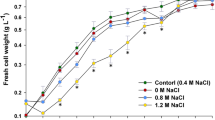
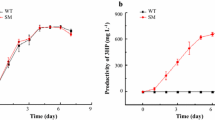
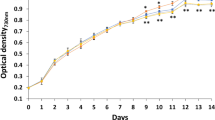
Abstract
Synechocystis strains are considered as valuable resources for production of useful metabolites. Comparative primary metabolic and lipidomic profiling of freshwater and marine Synechocystis strains is needed to understand the physiological characteristics of each strain at molecular level. In this study, we performed comparative primary metabolic and lipidomic profiling of freshwater Synechocystis sp. PCC 6803 (hereafter Synechocystis 6803) and marine Synechocystis sp. PCC 7338 (hereafter Synechocystis 7338) by using gas chromatography-mass spectrometry and nano electrospray ionization-mass spectrometry. Neophytadiene, phytol, monogalactosyldiacylglycerol (MGDG), and digalactosyldiacylglycerol (DGDG) were significantly higher in Synechocystis 6803. Levels of alanine, aspartic acid, valine, linoleic acid, linolenic acid, oleic acid, plamitic acid, stearic acid, glucosylglycerol, sucrose, phosphatidylglycerol (PG), and diacylglyceryltrimethylhomoserine (DGTS) (only detected in Synechocystis 7338) were significantly higher in Synechocystis 7338. Our results provide basic information to choose Synechocystis strains for various industrial usages, and also provide fundamental information on the future development of novel Synechocystis strains to produce high levels of specific metabolites by metabolic pathway modulation.
Similar content being viewed by others
References
Parmar, A., N. K. Singh, A. Pandey, E. Gnansounou, and D. Madamwar (2011) Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 102: 10163–10172.
Gouveia, L. and A. C. Oliveira (2009) Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 36: 269–274.
Griffiths, M. J. and S. T. L. Harrison (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 21: 493–507.
Lee, S. J., S. Go, G. T. Jeong, and S. K. Kim (2011) Oil production from five marine microalgae for the production of biodiesel. Biotechnol. Bioprocess Eng. 16: 561–566.
Wijffels, R. H., O. Kruse, and K. J. Hellingwerf (2013) Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 24: 405–413.
Dexter, J. and P. Fu (2009) Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci. 2: 857–864.
Tan, X., L. Yao, Q. Gao, W. Wang, F. Qi, and X. Lu (2011) Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab. Eng. 13: 169–176.
Liu, X., J. Sheng, and R. Curtiss III (2011) Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA. 108: 6899–6904.
Yu, Y., L. You, D. Liu, W. Hollinshead, Y. J. Tang, and F. Zhang (2013) Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar. Drugs 11: 2894–2916.
Institut Psteur, https://Catalogue-crbip.pasteur.fr.
Park, B. I., S. J. Hong, B. K. Cho, D. M. Kim, H. Lee, H. K. Choi, and C. G. Lee (2019) Optimization of culture conditions for enhanced phycobiliprotein production in Synechocystis sp. PCC 7338. Abstracts of the 59th KSIEC Meeting. May 1-3. Busan, Korea.
Okazaki, Y. and K. Saito (2012) Recent advances of metabolomics in plant biotechnology. Plant Biotechnol. Rep. 6: 1–15.
Fiehn, O. (2002) Metabolomics-the link between genotypes and phenotypes. Plant Mol. Biol. 48: 155–171.
Barofsky, A., C. Vidoudez, and G. Pohnert (2009) Metabolic profiling reveals growth stage variability in diatom exudates. Limnol. Oceanogr. Methods. 7: 382–390.
Rezanka, T., L. Nedbalova, J. Lukavsky, L. Prochazkova, and K. Sigler (2017) Lipidomic analysis of two closely related strains of the microalga Parietochloris (Trebouxiophyceae, Chlorophyta). Algal Res. 25:473–482.
Kim, S. H., H. M. Aim, S. R. Lim, S. J. Hong, B. K. Cho, H. Lee, C. G. Lee, and H. K. Choi (2015) Comparative lipidomic profiling of two Dunaliella tertiolecta strains with different growth temperatures under nitrate-deficient conditions. J. Agric. Food Chem. 63: 880–887.
Han, X. and R. W. Gross (2003) Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 44: 1071–1079.
Li, L., J. Han, Z. Wang, J. Liu, J. Wei, S. Xiong, and Z. Zhao (2014) Mass spectrometry methodology in lipid analysis. Int. J. Mol. Sci. 15: 10492–10507.
Wang, Y., M. Shi, X. Niu, X. Zhang, L. Gao, L. Chen, J. Wang, and W. Zhang (2014) Metabolomic basis of laboratory evolution of butanol tolerance in photosynthetic Synechocystis sp. PCC 6803. Mircob. Cell Fact. 13: 151.
Belghit, I., J. D. Rasinger, S. Heesch, I. Biancarosa, N. Liland, B. Torstensen, R. Waagbø, E. J. Lock, and C. G. Bruckner (2017) In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 26: 240–249.
Shukla, E., S. S. Singh, P. Singh, and A. K. Mishra (2012) Chemotaxonomy of heterocystous cyanobacteria using FAME profiling as species markers. Protoplasma. 249: 651–661.
Li, S., J. Xu, Y. Jiang, C. Zhou, X. Yu, Y. Zhong, J. Chen, and X. Yan (2015) Lipidomic analysis can distinguish between two morphologically similar strains of Nannochloropsis oceanica. J. Phycol. 51: 264–276.
Velasquez-Orta, S. B., J. G. M. Lee, and A. P. Harvey (2013) Evaluation of FAME production from wet marine and freshwater microalgae by in situ transesterification. Biochem. Eng. J. 76: 83–89.
Talebi, A. F., M. Tabatabaei, S. K. Mohtashami, M. Tohidfar, and F. Moradi (2013) Comparative salt stress study on intracellular ion concentration in marine and salt-adapted freshwater strains of microalgae. Not. Sci. Biol. 5: 309–315.
Lee, H. S., Z. H. Kim, H. Park, and C. G. Lee (2016) Specific light uptake rates can enhance astaxanthin productivity in Haematococcus lacustris. Bioprocess Biosyst. Eng. 39: 815–823.
Kim, J. Y., H. Y. Kim, J. Y. Jeon, D. M. Kim, Y. Zhou, J. S. Lee, H. Lee, and H. K. Choi (2017) Effects of coronatine elicitation on growth and metabolic profiles of Lemna paucicostata culture. PLoS One. 12: e0187622.
Kim, S. H., S. R. Lim, S. J. Hong, B. K. Cho, H. Lee, C. G. Lee, and H. K. Choi (2016) Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J. Agric. Food Chem. 64: 4807–4816.
Kind, T., K. H. Liu, D. Y. Lee, B. DeFelice, J. K. Meissen, and F. Oliver (2013) LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods. 10: 755–758.
Eriksson, L. (2006) Multi- and Megavariate Data Analysis. 2nd ed., pp. 39–102. Umetrics Academy, Umea, Sweden.
Schrader, A., W. Siefken, T. Kueper, U. Breitenbach, C. Gatermann, G. Sperling, T. Biernoth, C. Schemer, F. Stab, H. Wenck, K. P. Wittern, and T. Blatt (2012) Effects of glyceryl glucoside on AQP3 expression, barrier function and hydration of human skin. Skin Pharmacol. Physiol. 25: 192–199.
Reed, R. H. and W. D. P. Stewart (1985) Osmotic adjustment and organic solute accumulation in unicellular cyanobacteria from freshwater and marine habitats. Mar. Biol. 88: 1–9.
Ochsenkuhn, M. A., T. Röthig, C. D'Angelo, J. Wiedenmann, and C. R. Voolstra (2017) The role of floridoside in osmoadaptation of coral-associated algal endosymbionts to high-salinity conditions. Sci. Adv. 3: e1602047.
Tan, X., Q. Luo, and X. Lu (2016) Biosynthesis, biotechnological production, and applications of glucosylglycerols. Appl. Microbiol. Biotechnol. 100: 6131–6139.
Fulda, S., S. Mikkat, F. Huang, I. Huckauf, K. Marin, B. Norling, and M. Hagemann (2006) Proteome analysis of salt stress response in the cyanobacterium Synechocystis sp. strain PCC 6803. Proteomics. 6: 2733–2745.
Leuchtenberger, W., K. Huthmacher, and K. Drauz (2005) Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 69: 1–8.
Ijima, H., Y. Nakaya, A. Kuwahara, M. Y. Hirai, and T. Osanai (2015) Seawater cultivation of freshwater cyanobacterium Synechocystis sp. PCC 6803 drastically alters amino acid composition and glycogen metabolism. Front. Microbiol. 6: 326.
Delauney, A. J. and D. P. S. Verma (1993) Proline biosynthesis and osmoregulation in plants. Plant J. 4: 215–223.
Guerzoni, J. T. S., N. G. Belintani, R. M. P. Moreira, A. A. Hoshino, D. S. Domingues, J. C. B. Filho, and L. G. E. Vieira (2014) Stress-induced Al-pyrroline-5-carboxylate synthetase (P5CS) gene confers tolerance to salt stress in transgenic sugarcane. Acta Physiol. Plant. 36: 2309–2319.
Kumar, S. G., A. M. Reddy, and C. Sudhakar (2003) NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci. 165: 1245–1251.
Allakhverdiev, S. I., M. Kinoshita, M. Inaba, I. Suzuki, and N. Murata (2002) Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol. 125: 1842–1853.
Dieterle, M. and E. Schwab (2016) Raw material change in the chemical industry. Top. Catal. 59: 817–822.
Singh, R., S. A. Dar, and P. Sharma (2012) Antibacterial activity and toxicological evaluation of semi purified hexane extract of Urtica dioica leaves. Res. J. Med. Plant. 6: 123–135.
Lee, W., E. R. Woo, and D. G. Lee (2016) Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic. Res. 50: 1309–1318.
McGinty, D., C. S. Letizia, and A. M. Api (2010) Fragrance material review on phytol. Food Chem. Toxicol. 48: S59–S63.
Plouguerné, E., L. M. De Souza, G. L. Sassaki, J. F. Cavalcanti, M. T. V. Romanos, B. A. P. Da Gama, R. C. Pereira, and E. Barreto-Bergter (2013) Antiviral sulfoquinovosyldiacylglycerols (SQDGs) from the brazilian brown seaweed Sargassum vulgare. Mar. Drugs. 11: 4628–4640.
Tsai, C. J. and B. S. Pan (2012) Identification of sulfoglycolipid bioactivities and characteristic fatty acids of marine macroalgae. J. Agric. Food Chem. 60: 8404–8410.
Aoki, M., N. Sato, A. Meguro, and M. Tsuzuki (2004) Differing involvement of sulfoquinovosyl diacylglycerol in photosystem II in two species of unicellular cyanobacteria. Eur. J. Biochem. 271: 685–693.
Elkahoui, S., A. Smaoui, M. Zarrouk, R. Ghrir, and F. Limam (2004) Salt-induced lipid changes in Catharanthus roseus cultured cell suspensions. Phytochemistry. 65: 1911–1917.
Antonopoulou, S., T. Nomikos, A. Oikonomou, A. Kyriacou, M. Andriotis, E. Fragopoulou, and A. Pantazidou (2005) Characterization of bioactive glycolipids from Scytonema julianum (cyanobacteria). Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 140: 219–231.
Sui, N., D. Wei, F. Chen, and S. T. Yang (2012) Lipidomic profiling and discovery of lipid biomarkers in snow alga Chlamydomonas nivalis under salt stress. Eur. J. Lipid Sci. Technol. 114: 253–265.
Sui, N., M. Li, K. Li, J. Song, and B. S. Wang (2010) Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica. 48: 623–629.
Banskota, A. H., R. Stefanova, S. Sperker, and P. J. McGinn (2013) New diacylglyceryltrimethylhomoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J. Appl. Phycol. 25: 1513–1521.
Sato, N. and M. Furuya (1984) Distribution of diacyl-glyceryltrimethylhomoserine in selected species of vascular plants. Phytochemistiry. 23: 1625–1627.
Rezanka, T., I. Viden, J. V. Go, I. Dor, and V. M. Dembitsky (2003) Polar lipids and fatty acids of three wild cyanobacterial strains of the genus Chroococcidiopsis. Folia Microbiol. 48: 781–786.
Dembitsky, V. M. (1996) Betaine ether-linked glycerolipids: Chemistry and biology. Prog. Lipid Res. 35: 1–51.
Sato, N. (1992) Betaine lipids. Bot. Mag. Tokyo. 105: 185–197.
Banskota, A. H., R. Stefanova, P. Gallant, and P. J. McGinn (2013) Mono- and digalactosyldiacylglycerols: Potent nitric oxide inhibitors from the marine microalga Nannochloropsis granulata. J. Appl. Phycol. 25: 349–357.
Kobayashi, K., K. Endo, and H. Wada (2016) Roles of lipids in photosynthesis, pp. 21–49. In: Y. Nakamura and Y. Li-Beisson (eds.). Lipids in Plant and Algae Development. Springer International Publishing, Berlin, Germany.
Sudhir, P. R., D. Pogoryelov, L. Kovacs, G. Garab, and S. D. S. Murthy (2005) The effects of salt stress on photosynthetic electron transport and thylakoid membrane proteins in the cyanobacterium Spirulina platensis. J Biochem Mol Biol. 38: 481–485.
Parida, A. K., A. B. Das, and B. Mittra (2003) Effects of NaCl stress on the structure, pigment complex composition, and photosynthetic activity of mangrove Bruguiera parviflora chloroplasts. Photosynthetica. 41: 191.
Acknowledgments
This work was supported by the Basic Core Technology Development Program for the Oceans and the Polar Regions of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (No. NRF-2016M1A5A1027464), National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) [NRF-2015R1A5A1008958], and by the Chung-Ang University Graduate Research Scholarship in 2018. The authors declare no conflict of interest. Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
12257_2019_432_MOESM1_ESM.pdf
Comparative Primary Metabolic and Lipidomic Profiling of Freshwater and Marine Synechocystis Strains Using by GC-MS and NanoESI-MS Analyses
Rights and permissions
About this article
Cite this article
Noh, Y., Lee, H., Hong, SJ. et al. Comparative Primary Metabolic and Lipidomic Profiling of Freshwater and Marine Synechocystis Strains Using by GC-MS and NanoESI-MS Analyses. Biotechnol Bioproc E 25, 308–319 (2020). https://doi.org/10.1007/s12257-019-0432-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0432-8